Selective serotonin reuptake inhibitor
| Selective serotonin reuptake inhibitor | |
|---|---|
| Drug class | |
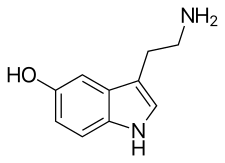
Serotonin, the neurotransmitter that is involved in the mechanism of action of SSRIs.
| |
| Class identifiers | |
| Synonyms | Serotonin-specific reuptake inhibitors, serotonergic antidepressants[1] |
| Use | Major depressive disorder, anxiety disorders |
| ATC code | N06AB |
| Biological target | Serotonin transporter |
| Clinical data | |
| Drugs.com | Drug Classes |
| Consumer Reports | Best Buy Drugs |
| External links | |
| MeSH | D017367 |
| In Wikidata | |
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder and anxiety disorders.
The exact mechanism of action of SSRIs is unknown.[2] SSRIs are believed to increase the extracellular level of the neurotransmitterserotonin by limiting its reabsorption (reuptake) into the presynaptic cell, increasing the level of serotonin in the synaptic cleft available to bind to the postsynaptic receptor. They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having only weak affinity for the norepinephrine and dopamine transporters.
SSRIs are the most widely prescribed antidepressants in many countries.[3] The efficacy of SSRIs in mild or moderate cases of depression has been disputed,[4][5][6] and may be outweighed by side effects.[7]
Medical uses[edit]
The main indication for SSRIs is major depressive disorder (also called "major depression", "clinical depression" and often simply "depression"). SSRIs are frequently prescribed for anxiety disorders, such as social anxiety disorder, panic disorders, obsessive–compulsive disorder (OCD), eating disorders, chronic pain and occasionally, for posttraumatic stress disorder (PTSD). They are also frequently used to treat depersonalization disorder, although generally with poor results.[8]
Depression[edit]
Antidepressants are recommended by the National Institute for Health and Care Excellence (NICE) as a first-line treatment of severe depression and for the treatment of mild-to-moderate depression that persists after conservative measures such as cognitive therapy.[9] They recommend against their routine use in those who have chronic health problems and mild depression.[9]
There has been controversy regarding the efficacy of antidepressants in treating depression depending on its severity and duration.
- Two meta-analyses published in 2008 (Kirsch) and 2010 (Fournier) found that in mild and moderate depression, the effect of SSRIs is small or none compared to placebo, while in very severe depression the effect of SSRIs is between "relatively small" and "substantial".[4][10] The 2008 meta-analysis combined 35 clinical trials submitted to the Food and Drug Administration (FDA) before licensing of four newer antidepressants (including the SSRIs paroxetine and fluoxetine, the non-SSRI antidepressant nefazodone, and the serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine). The authors attributed the relationship between severity and efficacy to a reduction of the placebo effect in severely depressed patients, rather than an increase in the effect of the medication.[10] Some researchers have questioned the statistical basis of this study suggesting that it underestimates the effect size of antidepressants.[11][12]
- A 2010 comprehensive review conducted by NICE concluded that antidepressants have no advantage over placebo in the treatment of short-term mild depression, but that the available evidence supported the use of antidepressants in the treatment of dysthymia and other forms of chronic mild depression.[13]
- A 2012 meta-analysis of fluoxetine and venlafaxine concluded that statistically and clinically significant treatment effects were observed for each drug relative to placebo irrespective of baseline depression severity.[14]
- In 2014 the U.S. FDA published a systematic review of all antidepressant maintenance trials submitted to the agency between 1985 and 2012. The authors concluded that maintenance treatment reduced the risk of relapse by 52% compared to placebo, and that this effect was primarily due to recurrent depression in the placebo group rather than a drug withdrawal effect.[15]
- A 2017 systematic review stated that "SSRIs versus placebo seem to have statistically significant effects on depressive symptoms, but the clinical significance of these effects seems questionable and all trials were at high risk of bias. Furthermore, SSRIs versus placebo significantly increase the risk of both serious and non-serious adverse events. Our results show that the harmful effects of SSRIs versus placebo for major depressive disorder seem to outweigh any potentially small beneficial effects".[7] The review was criticized for being inaccurate and misleading.[16]
- In 2018 a systematic review of 21 different antidepressants found that all analysed antidepressants were more efficacious than placebo in adults with major depressive disorder.[17] Effect sizes measured at 8-weeks after treatment onset however were modest.[17]
There does not appear to be a big difference in the effectiveness between medications in the second generation antidepressants (SSRIs and SNRIs).[18]
In children there are concerns around the quality of the evidence on the meaningfulness of benefits seen.[19] If a medication is used, fluoxetine appears to be first line.[19]
Generalized anxiety disorder[edit]
SSRIs are recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of generalized anxiety disorder (GAD) that has failed to respond to conservative measures such as education and self-help activities. GAD is a common disorder of which the central feature is excessive worry about a number of different events. Key symptoms include excessive anxiety about multiple events and issues, and difficulty controlling worrisome thoughts that persists for at least 6 months.
Antidepressants provide a modest-to-moderate reduction in anxiety in GAD,[20] and are superior to placebo in treating GAD.[21] The efficacy of different antidepressants is similar.[20][21]
Obsessive–compulsive disorder[edit]
SSRIs are a second line treatment of adult obsessive–compulsive disorder (OCD) with mild functional impairment and as first line treatment for those with moderate or severe impairment. In children, SSRIs can be considered a second line therapy in those with moderate-to-severe impairment, with close monitoring for psychiatric adverse effects.[22] SSRIs are efficacious in the treatment of OCD; patients treated with SSRIs are about twice as likely to respond to treatment as those treated with placebo.[23][24] Efficacy has been demonstrated both in short-term treatment trials of 6 to 24 weeks and in discontinuation trials of 28 to 52 weeks duration.[25][26][27]
Eating disorders[edit]
Anti-depressants are recommended as an alternative or additional first step to self-help programs in the treatment of bulimia nervosa.[28] SSRIs (fluoxetine in particular) are preferred over other anti-depressants due to their acceptability, tolerability, and superior reduction of symptoms in short-term trials. Long-term efficacy remains poorly characterized.
Similar recommendations apply to binge eating disorder.[28] SSRIs provide short-term reductions in binge eating behavior, but have not been associated with significant weight loss.[29]
Clinical trials have generated mostly negative results for the use of SSRIs in the treatment of anorexia nervosa.[30] Treatment guidelines from the National Institute of Health and Clinical Excellence[28] recommend against the use of SSRIs in this disorder. Those from the American Psychiatric Association note that SSRIs confer no advantage regarding weight gain, but that they may be used for the treatment of co-existing depressive, anxiety, or OCD.[29]
Stroke recovery[edit]
SSRIs have been used in the treatment of stroke patients, including those with and without symptoms of depression. A recent meta-analysis of randomized, controlled clinical trials found a statistically significant effect of SSRIs on dependence, neurological deficit, depression, and anxiety. There was no statistically significant effect on death, motor deficits, or cognition.[31]
Premature ejaculation[edit]
SSRIs are effective for the treatment of premature ejaculation. Chronic administration is more efficacious than on demand use.[32]
Side effects[edit]
Side effects vary among the individual drugs of this class. However, certain types of adverse effects are found broadly among most if not all members of this class:
- increased risk of bone fractures by 1.7 fold[33]
- akathisia[34][35][36][37]
- suicidal ideation (thoughts of suicide) (see below)
- photosensitivity[38]
Sexual dysfunction[edit]
SSRIs can cause various types of sexual dysfunction such as anorgasmia, erectile dysfunction, diminished libido, genital numbness, and sexual anhedonia (pleasureless orgasm).[39]Sexual problems are common with SSRIs.[40] Poor sexual function is also one of the most common reasons people stop the medication.[41]
In some cases, symptoms of sexual dysfunction may persist after discontinuation of SSRIs.[39][42][43][44][45]
The mechanism by which SSRIs may cause sexual side effects is not well understood as of 2015. The range of possible mechanisms includes (1) nonspecific neurological effects (e.g., sedation) that globally impair behavior including sexual function; (2) specific effects on brain systems mediating sexual function; (3) specific effects on peripheral tissues and organs, such as the penis, that mediate sexual function; and (4) direct or indirect effects on hormones mediating sexual function.[46] Management strategies include: for erectile dysfunction the addition of a PDE5 inhibitor such as sildenafil; for decreased libido, possibly adding or switching to bupropion; and for overall sexual dysfunction, switching to nefazodone.[47]
A number of non-SSRI drugs are not associated with sexual side effects (such as bupropion, mirtazapine, tianeptine, agomelatine and moclobemide[48][49]).
Several studies have suggested that SSRIs may adversely affect semen quality.[50]
Cardiac[edit]
SSRIs do not appear to affect the risk of coronary heart disease (CHD) in those without a previous diagnosis of CHD.[52] A large cohort study suggested no substantial increase in the risk of cardiac malformations attributable to SSRI usage during the first trimester of pregnancy.[53] A number of large studies of people without known pre-existing heart disease have reported no EKG changes related to SSRI use.[54] The recommended maximum daily dose of citalopram and escitalopram was reduced due to concerns with QT intervalprolongation.[55][56][57] In overdose, fluoxetine has been reported to cause sinus tachycardia, myocardial infarction, junctional rhythms and trigeminy. Some authors have suggested electrocardiographic monitoring in patients with severe pre-existing cardiovascular disease who are taking SSRIs.[58]
Bleeding[edit]
SSRIs interact with anticoagulants, like warfarin, and antiplatelet drugs, like aspirin.[59][60][61][62] This includes an increased risk of GI bleeding, and post operative bleeding.[59] The relative risk of intracranial bleeding is increased, but the absolute risk is very low.[63] SSRIs are known to cause platelet dysfunction.[64][65] This risk is greater in those who are also on anticoagulants, antiplatelet agents and NSAIDs (nonsteroidal anti-inflammatory drugs), as well as with the co-existence of underlying diseases such as cirrhosis of the liver or liver failure.[66][67]
Fracture risk[edit]
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests an association between SSRI usage at therapeutic doses and a decrease in bone mineral density, as well as increased fracture risk,[68][69][70][71] a relationship that appears to persist even with adjuvant bisphosphonate therapy.[72] However, because the relationship between SSRIs and fractures is based on observational data as opposed to prospective trials, the phenomenon is not definitively causal.[73] There also appears to be an increase in fracture-inducing falls with SSRI use, suggesting the need for increased attention to fall risk in elderly patients using the medication.[73] The loss of bone density does not appear to occur in younger patients taking SSRIs.[74]
Discontinuation syndrome[edit]
Serotonin reuptake inhibitors should not be abruptly discontinued after extended therapy, and whenever possible, should be tapered over several weeks to minimize discontinuation-related symptoms which may include nausea, headache, dizziness, chills, body aches, paresthesias, insomnia, and electric shock-like sensations. Paroxetine may produce discontinuation-related symptoms at a greater rate than other SSRIs, though qualitatively similar effects have been reported for all SSRIs.[75][76] Discontinuation effects appear to be less for fluoxetine, perhaps owing to its long half-life and the natural tapering effect associated with its slow clearance from the body. One strategy for minimizing SSRI discontinuation symptoms is to switch the patient to fluoxetine and then taper and discontinue the fluoxetine.[75]
Serotonin syndrome[edit]
Serotonin syndrome is typically caused by the use of two or more serotonergic drugs, including SSRIs.[77] Serotonin syndrome is a short-lived condition that can range from mild (most common) to deadly. Mild symptoms may consist of increased heart rate, shivering, sweating, dilated pupils, myoclonus (intermittent jerking or twitching), as well as overresponsive reflexes.[78] Concomitant use of an SSRI or selective norepinephrine reuptake inhibitor for depression with a triptan for migraine does not appear to heighten the risk of the serotonin syndrome.[79]
Suicide risk[edit]
Children and adolescents[edit]
Meta analyses of short duration randomized clinical trials have found that SSRI use is related to a higher risk of suicidal behavior in children and adolescents.[80][81][82] For instance, a 2004 U.S. Food and Drug Administration (FDA) analysis of clinical trials on children with major depressive disorder found statistically significant increases of the risks of "possible suicidal ideation and suicidal behavior" by about 80%, and of agitation and hostility by about 130%.[83] According to the FDA, the heightened risk of suicidality is within the first one to two months of treatment.[84][85][86] The National Institute for Health and Care Excellence (NICE) places the excess risk in the "early stages of treatment".[87] The European Psychiatric Association places the excess risk in the first two weeks of treatment and, based on a combination of epidemiological, prospective cohort, medical claims, and randomized clinical trial data, concludes that a protective effect dominates after this early period. A 2014 Cochrane review found that at six to nine months, suicidal ideation remained higher in children treated with antidepressants compared to those treated with psychological therapy.[86]
A recent comparison of aggression and hostility occurring during treatment with fluoxetine to placebo in children and adolescents found that no significant difference between the fluoxetine group and a placebo group.[88] There is also evidence that higher rates of SSRI prescriptions are associated with lower rates of suicide in children, though since the evidence is correlational, the true nature of the relationship is unclear.[89]
In 2004, the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom judged fluoxetine (Prozac) to be the only antidepressant that offered a favorable risk-benefit ratio in children with depression, though it was also associated with a slight increase in the risk of self-harm and suicidal ideation.[90] Only two SSRIs are licensed for use with children in the UK, sertraline (Zoloft) and fluvoxamine (Luvox), and only for the treatment of obsessive–compulsive disorder. Fluoxetine is not licensed for this use.[91]
Adults[edit]
It is unclear whether SSRIs affect the risk of suicidal behavior in adults.
- A 2005 meta-analysis of drug company data found no evidence that SSRIs increased the risk of suicide; however, important protective or hazardous effects could not be excluded.[92]
- A 2005 review observed that suicide attempts are increased in those who use SSRIs as compared to placebo and compared to therapeutic interventions other than tricyclic antidepressants. No difference risk of suicide attempts was detected between SSRIs versus tricyclic antidepressants.[93]
- On the other hand, a 2006 review suggests that the widespread use of antidepressants in the new "SSRI-era" appears to have led to a highly significant decline in suicide rates in most countries with traditionally high baseline suicide rates. The decline is particularly striking for women who, compared with men, seek more help for depression. Recent clinical data on large samples in the US too have revealed a protective effect of antidepressant against suicide.[94]
- A 2006 meta-analysis of random controlled trials suggests that SSRIs increase suicide ideation compared with placebo. However, the observational studies suggest that SSRIs did not increase suicide risk more than older antidepressants. The researchers stated that if SSRIs increase suicide risk in some patients, the number of additional deaths is very small because ecological studies have generally found that suicide mortality has declined (or at least not increased) as SSRI use has increased.[95]
- An additional meta-analysis by the FDA in 2006 found an age-related effect of SSRI's. Among adults younger than 25 years, results indicated that there was a higher risk for suicidal behavior. For adults between 25 and 64, the effect appears neutral on suicidal behavior but possibly protective for suicidal behavior for adults between the ages of 25 and 64. For adults older than 64, SSRI's seem to reduce the risk of both suicidal behavior.[80]
Pregnancy and breastfeeding[edit]
SSRI use in pregnancy has been associated with a variety of risks with varying degrees of proof of causation. As depression is independently associated with negative pregnancy outcomes, determining the extent to which observed associations between antidepressant use and specific adverse outcomes reflects a causative relationship has been difficult in some cases.[96] In other cases, the attribution of adverse outcomes to antidepressant exposure seems fairly clear.
SSRI use in pregnancy is associated with an increased risk of spontaneous abortion of about 1.7-fold.[97][98] Use is also associated preterm birth.[99]
A systematic review of the risk of major birth defects in antidepressant-exposed pregnancies found a small increase (3% to 24%) in the risk of major malformations and a risk of cardiovascular birth defects that did not differ from non-exposed pregnancies.[100] A study of fluoxetine-exposed pregnancies found a 12% increase in the risk of major malformations that just missed statistical significance.[101] Other studies have found an increased risk of cardiovascular birth defects among depressed mothers not undergoing SSRI treatment, suggesting the possibility of ascertainment bias, e.g. that worried mothers may pursue more aggressive testing of their infants.[102] Another study found no increase in cardiovascular birth defects and a 27% increased risk of major malformations in SSRI exposed pregnancies.[98]
The FDA issued a statement on July 19, 2006 stating nursing mothers on SSRIs must discuss treatment with their physicians. However, the medical literature on the safety of SSRIs has determined that some SSRIs like Sertraline and Paroxetine are considered safe for breastfeeding.[103][104][105]
Neonatal abstinence syndrome[edit]
Several studies have documented neonatal abstinence syndrome, a syndrome of neurological, gastrointestinal, autonomic, endocrine and/or respiratory symptoms among a large minority of infants with intrauterine exposure. These syndromes are short-lived, but insufficient long-term data is available to determine whether there are long-term effects.[106][107]
Persistent pulmonary hypertension[edit]
Persistent pulmonary hypertension (PPHN) is a serious and life-threatening, but very rare, lung condition that occurs soon after birth of the newborn. Newborn babies with PPHN have high pressure in their lung blood vessels and are not able to get enough oxygen into their bloodstream. About 1 to 2 babies per 1000 babies born in the U.S. develop PPHN shortly after birth, and often they need intensive medical care. It is associated with about a 25% risk of significant long-term neurological deficits.[108] A 2014 meta analysis found no increased risk of persistent pulmonary hypertension associated with exposure to SSRI's in early pregnancy and a slight increase in risk associates with exposure late in pregnancy; "an estimated 286 to 351 women would need to be treated with an SSRI in late pregnancy to result in an average of one additional case of persistent pulmonary hypertension of the newborn.".[109] A review published in 2012 reached conclusions very similar to those of the 2014 study.[110]
Neuropsychiatric effects in offspring[edit]
According to a 2015 review available data found that "some signal exists suggesting that antenatal exposure to SSRIs may increase the risk of ASDs (autism spectrum disorders)"[111] even though a large cohort study published in 2013[112] and a cohort study using data from Finland's national register between the years 1996 and 2010 and published in 2016 found no significant association between SSRI use and autism in offspring.[113] The 2016 Finland study also found no association with ADHD, but did find an association with increased rates of depression diagnoses in early adolescence.[113]
Overdose[edit]
SSRIs appear safer in overdose when compared with traditional antidepressants, such as the tricyclic antidepressants. This relative safety is supported both by case series and studies of deaths per numbers of prescriptions.[114] However, case reports of SSRI poisoning have indicated that severe toxicity can occur[115] and deaths have been reported following massive single ingestions,[116] although this is exceedingly uncommon when compared to the tricyclic antidepressants.[114]
Because of the wide therapeutic index of the SSRIs, most patients will have mild or no symptoms following moderate overdoses. The most commonly reported severe effect following SSRI overdose is serotonin syndrome; serotonin toxicity is usually associated with very high overdoses or multiple drug ingestion.[117] Other reported significant effects include coma, seizures, and cardiac toxicity.[114]
The SSRIs, in decreasing toxicity in overdose, can be listed as follows: > citalopram (due to the potential for QT interval prolongation) > fluvoxamine > escitalopram > paroxetine > sertraline > fluoxetine.[118]
Interactions[edit]
- Linezolid
- Monoamine oxidase inhibitors (MAOIs) including moclobemide, phenelzine, tranylcypromine, selegiline and methylene blue
- Lithium
- Sibutramine
- MDMA (ecstasy)
- Dextromethorphan
- Tramadol
- Pethidine/meperidine
- St. John's wort
- Yohimbe
- Tricyclic antidepressants (TCAs)
- Serotonin-norepinephrine reuptake inhibitors (SNRIs)
- Buspirone
- Triptan
- Mirtazapine
Painkillers of the NSAIDs drug family may interfere and reduce efficiency of SSRIs and may compound the increased risk of gastrointestinal bleeds caused by SSRI use.[60][62][121]NSAIDs include:
There are a number of potential pharmacokinetic interactions between the various individual SSRIs and other medications. Most of these arise from the fact that every SSRI has the ability to inhibit certain P450 cytochromes.[122][123]
| Drug name | CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4 | CYP2B6 |
|---|---|---|---|---|---|---|
| Citalopram | + | 0 | 0 | + | 0 | 0 |
| Escitalopram | 0 | 0 | 0 | + | 0 | 0 |
| Fluoxetine | + | ++ | +/++ | +++ | + | + |
| Fluvoxamine | +++ | ++ | +++ | + | + | + |
| Paroxetine | + | + | + | +++ | + | +++ |
| Sertraline | + | + | +/++ | + | + | + |
Legend:
0 — no inhibition
+ — mild inhibition
++ — moderate inhibition
+++ — strong inhibition
0 — no inhibition
+ — mild inhibition
++ — moderate inhibition
+++ — strong inhibition
List of SSRIs[edit]
Marketed[edit]
Antidepressants[edit]
- Citalopram (Celexa)
- Escitalopram (Lexapro)
- Fluoxetine (Prozac)
- Fluvoxamine (Luvox)
- Paroxetine (Paxil)
- Sertraline (Zoloft)
show
Structures
Others[edit]
- Dapoxetine (Priligy)
show
Structures
Discontinued[edit]
Antidepressants[edit]
- Indalpine (Upstène)
- Zimelidine (Zelmid)
show
Structures
Never marketed[edit]
Antidepressants[edit]
- Alaproclate (GEA-654)
- Centpropazine
- Cericlamine (JO-1017)
- Femoxetine (Malexil; FG-4963)
- Ifoxetine (CGP-15210)
- Omiloxetine
- Panuramine (WY-26002)
- Pirandamine (AY-23713)
- Seproxetine ((S)-norfluoxetine)
show
Structures
Related drugs[edit]
Although described as SNRIs, duloxetine (Cymbalta), venlafaxine (Effexor), and desvenlafaxine (Pristiq) are in fact relatively selective as serotonin reuptake inhibitors (SRIs).[124]They are about at least 10-fold selective for inhibition of serotonin reuptake over norepinephrine reuptake.[124] The selectivity ratios are approximately 1:30 for venlafaxine, 1:9 for duloxetine, and 1:14 for desvenlafaxine.[124] At low doses, these SNRIs act mostly as SSRIs; only at higher doses do they also prominently inhibit norepinephrine reuptake.[125][126]Milnacipran (Ixel, Savella) and its stereoisomer levomilnacipran (Fetzima) are the only widely marketed SNRIs that inhibit serotonin and norepinephrine to similar degrees, both with ratios close to 1:1.[124][127]
Vilazodone (Viibryd) and vortioxetine (Trintellix) are SRIs that also act as modulators of serotonin receptors and are described as serotonin modulators and stimulators (SMS).[128]Vilazodone is a 5-HT1A receptor partial agonist while vortioxetine is a 5-HT1A receptor agonist and 5-HT3 and 5-HT7 receptor antagonist.[128] Litoxetine (SL 81-0385) and lubazodone(YM-992, YM-35995) are similar drugs that were never marketed.[129][130][131][132] They are SRIs and litoxetine is also a 5-HT3 receptor antagonist[129][130] while lubazodone is also a 5-HT2A receptor antagonist.[131][132]
Chlorphenamine (Chlor-Trimeton) is an over-the-counter antihistamine that also acts as a potent and selective SRI (KD = 15.2 nM).[133][134] It has been suggested for potential use as an over-the-counter antidepressant.[134] The now-withdrawn SSRI zimelidine was derived from chlorphenamine.[134]
Mechanism of action[edit]
Serotonin reuptake inhibition[edit]
In the brain, messages are passed from a nerve cell to another via a chemical synapse, a small gap between the cells. The presynaptic cell that sends the information releases neurotransmitters including serotonin into that gap. The neurotransmitters are then recognized by receptors on the surface of the recipient postsynaptic cell, which upon this stimulation, in turn, relays the signal. About 10% of the neurotransmitters are lost in this process; the other 90% are released from the receptors and taken up again by monoamine transporters into the sending presynaptic cell, a process called reuptake.
SSRIs inhibit the reuptake of serotonin. As a result, the serotonin stays in the synaptic gap longer than it normally would, and may repeatedly stimulate the receptors of the recipient cell. In the short run, this leads to an increase in signaling across synapses in which serotonin serves as the primary neurotransmitter. On chronic dosing, the increased occupancy of post-synaptic serotonin receptors signals the pre-synaptic neuron to synthesize and release less serotonin. Serotonin levels within the synapse drop, then rise again, ultimately leading to downregulation of post-synaptic serotonin receptors.[135] Other, indirect effects may include increased norepinephrine output, increased neuronal cyclic AMP levels, and increased levels of regulatory factors such as BDNF and CREB.[136] Owing to the lack of a widely accepted comprehensive theory of the biology of mood disorders, there is no widely accepted theory of how these changes lead to the mood-elevating and anti-anxiety effects of SSRIs.[citation needed]
Sigma receptor ligands[edit]
| Medication | SERT | σ1 | σ2 | σ1 / SERT | |
|---|---|---|---|---|---|
| Citalopram | 1.16 | 292–404 | Agonist | 5,410 | 252–348 |
| Escitalopram | 2.5 | 288 | Agonist | ND | ND |
| Fluoxetine | 0.81 | 191–240 | Agonist | 16,100 | 296–365 |
| Fluvoxamine | 2.2 | 17–36 | Agonist | 8,439 | 7.7–16.4 |
| Paroxetine | 0.13 | ≥1,893 | ND | 22,870 | ≥14,562 |
| Sertraline | 0.29 | 32–57 | Antagonist | 5,297 | 110–197 |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||||
In addition to their actions as reuptake inhibitors of serotonin, some SSRIs are also, coincidentally, ligands of the sigma receptors.[137][138] Fluvoxamine is an agonist of the σ1 receptor, while sertraline is an antagonist of the σ1 receptor, and paroxetine does not significantly interact with the σ1 receptor.[137][138] None of the SSRIs have significant affinity for the σ2 receptor, and the SNRIs, unlike the SSRIs, do not interact with either of the sigma receptors.[137][138] Fluvoxamine has by far the strongest activity of the SSRIs at the σ1 receptor.[137][138]High occupancy of the σ1 receptor by clinical dosages of fluvoxamine has been observed in the human brain in positron emission tomography (PET) research.[137][138] It is thought that agonism of the σ1 receptor by fluvoxamine may have beneficial effects on cognition.[137][138] In contrast to fluvoxamine, the relevance of the σ1 receptor in the actions of the other SSRIs is uncertain and questionable due to their very low affinity for the receptor relative to the SERT.[citation needed]
Anti-inflammatory effects[edit]
The role of inflammation and the immune system in depression has been extensively studied. The evidence supporting this link has been shown in numerous studies over the past ten years. Nationwide studies and meta-analyses of smaller cohort studies have uncovered a correlation between pre-existing inflammatory conditions such as type 1 diabetes, rheumatoid arthritis (RA), or hepatitis, and an increased risk of depression. Data also shows that using pro-inflammatory agents in the treatment of diseases like melanoma can lead to depression. Several meta-analytical studies have found increased levels of proinflammatory cytokines and chemokines in depressed patients.[139] This link has led scientists to investigate the effects of antidepressants on the immune system.
SSRIs were originally invented with the goal of increasing levels of available serotonin in the extracellular spaces. However, the delayed response between when patients first begin SSRI treatment to when they see effects has led scientists to believe that other molecules are involved in the efficacy of these drugs.[140] To investigate the apparent anti-inflammatory effects of SSRIs, both Kohler et al. and Więdłocha et al. conducted meta-analyses which have shown that after antidepressant treatment the levels of cytokines associated with inflammation are decreased.[141][142] A large cohort study conducted by researchers in the Netherlands investigated the association between depressive disorders, symptoms, and antidepressants with inflammation. The study showed decreased levels of interleukin (IL)-6, a cytokine that has proinflammatory effects, in patients taking SSRIs compared to non-medicated patients.[143]
Treatment with SSRIs has shown reduced production of inflammatory cytokines such as IL-1β, tumor necrosis factor (TNF)-α, IL-6, and interferon (IFN)-γ, which leads to a decrease in inflammation levels and subsequently a decrease in the activation level of the immune response.[144] These inflammatory cytokines have been shown to activate microglia which are specialized macrophages that reside in the brain. Macrophages are a subset of immune cells responsible for host defense in the innate immune system. Macrophages can release cytokines and other chemicals to cause an inflammatory response. Peripheral inflammation can induce an inflammatory response in microglia and can cause neuroinflammation. SSRIs inhibit proinflammatory cytokine production which leads to less activation of microglia and peripheral macrophages. SSRIs not only inhibit the production of these proinflammatory cytokines, they also have been shown to upregulate anti-inflammatory cytokines such as IL-10. Taken together, this reduces the overall inflammatory immune response.[144][145]
In addition to affecting cytokine production, there is evidence that treatment with SSRIs has effects on the proliferation and viability of immune system cells involved in both innate and adaptive immunity. Evidence shows that SSRIs can inhibit proliferation in T-cells, which are important cells for adaptive immunity and can induce inflammation. SSRIs can also induce apoptosis, programmed cell death, in T-cells. The full mechanism of action for the anti-inflammatory effects of SSRIs is not fully known. However, there is evidence for various pathways to have a hand in the mechanism. One such possible mechanism is the increased levels of cyclic adenosine monophosphate (cAMP) as a result of interference with activation of protein kinase A (PKA), a cAMP dependent protein. Other possible pathways include interference with calcium ion channels, or inducing cell death pathways like MAPK.[146]
The anti-inflammatory effects of SSRIs have prompted studies of the efficacy of SSRIs in the treatment of autoimmune diseases such as multiple sclerosis, RA, inflammatory bowel diseases, and septic shock. These studies have been performed in animal models but have shown consistent immune regulatory effects. Fluoxetine, an SSRI, has also shown efficacy in animal models of graft vs. host disease.[146] SSRIs have also been used successfully as pain relievers in patients undergoing oncology treatment. The effectiveness of this has been hypothesized to be at least in part due to the anti-inflammatory effects of SSRIs.[145]
Pharmacogenetics[edit]
Large bodies of research are devoted to using genetic markers to predict whether patients will respond to SSRIs or have side effects that will cause their discontinuation, although these tests are not yet ready for widespread clinical use.[147]
Versus TCAs[edit]
SSRIs are described as 'selective' because they affect only the reuptake pumps responsible for serotonin, as opposed to earlier antidepressants, which affect other monoamine neurotransmitters as well, and as a result, SSRIs have fewer side effects.
There appears to be no significant difference in effectiveness between SSRIs and tricyclic antidepressants, which were the most commonly used class of antidepressants before the development of SSRIs.[148] However, SSRIs have the important advantage that their toxic dose is high, and, therefore, they are much more difficult to use as a means to commit suicide. Further, they have fewer and milder side effects. Tricyclic antidepressants also have a higher risk of serious cardiovascular side effects, which SSRIs lack.
SSRIs act on signal pathways such as cAMP (Cyclic AMP) on the postsynaptic neuronal cell, which leads to the release of Brain-Derived Neurotrophic Factor (BDNF). BDNF enhances the growth and survival of cortical neurons and synapses.[136]
History[edit]
Fluoxetine was introduced in 1987 and was the first major SSRI to be marketed.
Society and culture[edit]
Controversy[edit]
A study examining publication of results from FDA-evaluated antidepressants concluded that those with favorable results were much more likely to be published than those with negative results.[149]
David Healy has argued that warning signs were available for many years prior to regulatory authorities moving to put warnings on antidepressant labels that they might cause suicidal thoughts.[150] At the time these warnings were added, others argued that the evidence for harm remained unpersuasive[151][152] and others continued to do so after the warnings were added.[153][154]
See also[edit]
References[edit]
- ^ Barlow, David H. Durand, V. Mark (2009). "Chapter 7: Mood Disorders and Suicide". Abnormal Psychology: An Integrative Approach (Fifth ed.). Belmont, CA: Wadsworth Cengage Learning. p. 239. ISBN 0-495-09556-7. OCLC 192055408.
- ^ http://pi.lilly.com/us/prozac.pdf page 20
- ^ Preskorn SH, Ross R, Stanga CY (2004). "Selective Serotonin Reuptake Inhibitors". In Sheldon H. Preskorn, Hohn P. Feighner, Christina Y. Stanga, Ruth Ross. Antidepressants: Past, Present and Future. Berlin: Springer. pp. 241–62. ISBN 978-3-540-43054-4.
- ^ a b Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J (January 2010). "Antidepressant Drug Effects and Depression Severity". JAMA. 303 (1): 47–53. doi:10.1001/jama.2009.1943. PMC 3712503
 . PMID 20051569.
. PMID 20051569. - ^ Kramer, Peter (7 Sep 2011). "In Defense of Antidepressants". The New York Times. Retrieved 13 July 2011.
- ^ Pies R (April 2010). "Antidepressants Work, Sort of-Our System of Care Does Not". Journal of Clinical Psychopharmacology. 30 (2): 101–4. doi:10.1097/JCP.0b013e3181d52dea. PMID 20520282.
- ^ a b Jakobsen, Janus Christian; Katakam, Kiran Kumar; Schou, Anne; Hellmuth, Signe Gade; Stallknecht, Sandra Elkjær; Leth-Møller, Katja; Iversen, Maria; Banke, Marianne Bjørnø; Petersen, Iggiannguaq Juhl (2017-02-08). "Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis". BMC Psychiatry. 17. doi:10.1186/s12888-016-1173-2. ISSN 1471-244X. PMC 5299662
 . PMID 28178949.
. PMID 28178949. - ^ Medford, Nick. "Understanding and treating depersonalization disorder". Advances in Psychiatric Treatment (2005). Retrieved 2011-11-11.
- ^ a b "www.nice.org.uk" (PDF). Archived from the original (PDF) on September 28, 2013.
- ^ a b Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (February 2008). "Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration". PLoS Medicine. 5 (2): e45. doi:10.1371/journal.pmed.0050045. PMC 2253608
 . PMID 18303940.
. PMID 18303940. - ^ Horder J, Matthews P, Waldmann R (June 2010). "Placebo, Prozac and PLoS: significant lessons for psychopharmacology". Journal of Psychopharmacology. 25 (10): 1277–88. doi:10.1177/0269881110372544. PMID 20571143.
- ^ Fountoulakis KN, Möller HJ (August 2010). "Efficacy of antidepressants: a re-analysis and re-interpretation of the Kirsch data". The International Journal of Neuropsychopharmacology. 14 (3): 1–8. doi:10.1017/S1461145710000957. PMID 20800012.
- ^ Depression: The NICE Guideline on the Treatment and Management of Depression in Adults (Updated Edition) (PDF). RCPsych Publications. 2010. ISBN 1-904671-85-3.
- ^ Gibbons RD, Hur K, Brown CH, Davis JM, Mann JJ (June 2012). "Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine". Archives of General Psychiatry. 69 (6): 572–9. doi:10.1001/archgenpsychiatry.2011.2044. PMC 3371295
 . PMID 22393205.
. PMID 22393205. - ^ Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J (January 2010). "Antidepressant drug effects and depression severity: a patient-level meta-analysis". JAMA. 303 (1): 47–53. doi:10.1001/jama.2009.1943. PMC 3712503
 . PMID 20051569.
. PMID 20051569. - ^ Hieronymus, Fredrik; Lisinski, Alexander; Näslund, Jakob; Eriksson, Elias (July 2017). "Multiple possible inaccuracies cast doubt on a recent report suggesting selective serotonin reuptake inhibitors to be toxic and ineffective". Acta Neuropsychiatrica: 1–7. doi:10.1017/neu.2017.23. ISSN 0924-2708.
- ^ a b Cipriani, A; Furukawa, TA; Salanti, G; Chaimani, A; Atkinson, LZ; Ogawa, Y; Leucht, S; Ruhe, HG; Turner, EH; Higgins, JPT; Egger, M; Takeshima, N; Hayasaka, Y; Imai, H; Shinohara, K; Tajika, A; Ioannidis, JPA; Geddes, JR (20 February 2018). "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet. doi:10.1016/S0140-6736(17)32802-7. PMID 29477251.
- ^ Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Van Noord M, Mager U, Thieda P, Gaynes BN, Wilkins T, Strobelberger M, Lloyd S, Reichenpfader U, Lohr KN (December 2011). "Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis". Annals of Internal Medicine. 155(11): 772–85. doi:10.7326/0003-4819-155-11-201112060-00009. PMID 22147715.
- ^ a b Hetrick SE, McKenzie JE, Cox GR, Simmons MB, Merry SN (Nov 14, 2012). "Newer generation antidepressants for depressive disorders in children and adolescents". The Cochrane Database of Systematic Reviews. 11: CD004851. doi:10.1002/14651858.cd004851.pub3. PMID 23152227.
- ^ a b "www.nice.org.uk" (PDF). Retrieved 2013-02-20.
- ^ a b Kapczinski F, Lima MS, Souza JS, Schmitt R (2003). Kapczinski, Flavio FK, ed. "Antidepressants for generalized anxiety disorder". The Cochrane Database of Systematic Reviews (2): CD003592. doi:10.1002/14651858.CD003592. PMID 12804478.
- ^ "Obsessive-compulsive disorder: Core interventions in the treatment of obsessive-compulsive disorder and body dysmorphic disorder" (PDF). November 2005
- ^ Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, Kerse N, Macgillivray S (2009). Arroll, Bruce, ed. "Antidepressants versus placebo for depression in primary care". The Cochrane Database of Systematic Reviews (3): CD007954. doi:10.1002/14651858.CD007954. PMID 19588448.
- ^ Busko, Marlene (28 February 2008). "Review Finds SSRIs Modestly Effective in Short-Term Treatment of OCD". Medscape. Archived from the original on April 13, 2013.
- ^ Fineberg NA, Brown A, Reghunandanan S, Pampaloni I (2012). "Evidence-based pharmacotherapy of obsessive-compulsive disorder". The International Journal of Neuropsychopharmacology. 15 (8): 1173–91. doi:10.1017/S1461145711001829. PMID 22226028.
- ^ "Sertraline prescribing information" (PDF). Retrieved 2015-01-30.
- ^ "Paroxetine prescribing information" (PDF). Retrieved 2015-01-30.
- ^ a b c "Eating disorders in over 8s: management" (PDF). NICE. January 2004.
- ^ a b "National Guideline Clearinghouse | Practice guideline for the treatment of patients with eating disorders".
- ^ Flament MF, Bissada H, Spettigue W (March 2012). "Evidence-based pharmacotherapy of eating disorders". The International Journal of Neuropsychopharmacology. 15 (2): 189–207. doi:10.1017/S1461145711000381. PMID 21414249.
- ^ Mead GE, Hsieh CF, Lee R, Kutlubaev MA, Claxton A, Hankey GJ, Hackett ML (2012). Mead, Gillian E, ed. "Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery". The Cochrane Database of Systematic Reviews. 11: CD009286. doi:10.1002/14651858.CD009286.pub2. PMID 23152272.
- ^ Waldinger MD (November 2007). "Premature ejaculation: state of the art". The Urologic Clinics of North America. 34 (4): 591–9, vii–viii. doi:10.1016/j.ucl.2007.08.011. PMID 17983899.
- ^ Wu Q, Bencaz AF, Hentz JG, Crowell MD (January 2012). "Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies". Osteoporosis International. 23 (1): 365–75. doi:10.1007/s00198-011-1778-8. PMID 21904950.
- ^ Stahl SM, Lonnen AJ (2011). "The Mechanism of Drug-induced Akathsia". CNS Spectrums. PMID 21406165.
- ^ Lane RM (1998). "SSRI-induced extrapyramidal side-effects and akathisia: implications for treatment". Journal of Psychopharmacology. 12 (2): 192–214. doi:10.1177/026988119801200212. PMID 9694033.
- ^ Koliscak LP, Makela EH (2009). "Selective serotonin reuptake inhibitor-induced akathisia". Journal of the American Pharmacists Association. 49 (2): e28–36; quiz e37–8. doi:10.1331/JAPhA.2009.08083. PMID 19289334.
- ^ Leo RJ (1996). "Movement disorders associated with the serotonin selective reuptake inhibitors". The Journal of Clinical Psychiatry. 57 (10): 449–54. doi:10.4088/JCP.v57n1002. PMID 8909330.
- ^ September 23, 2012. "SSRIs and Depression". Emedicinehealth.com. Retrieved 2012-09-23.
- ^ a b Bahrick, Audrey (2008). "Persistence of Sexual Dysfunction Side Effects after Discontinuation of Antidepressant Medications: Emerging Evidence" (PDF). The Open Psychology Journal. 1: 42–50. doi:10.2174/1874350100801010042. Archived from the original (PDF) on 19 October 2013. Retrieved 30 January 2014.
- ^ Taylor, MJ; Rudkin, L; Bullemor-Day, P; Lubin, J; Chukwujekwu, C; Hawton, K (31 May 2013). "Strategies for managing sexual dysfunction induced by antidepressant medication". The Cochrane Database of Systematic Reviews. 5: CD003382. doi:10.1002/14651858.CD003382.pub3. PMID 23728643.
- ^ Kennedy, SH; Rizvi, S (April 2009). "Sexual dysfunction, depression, and the impact of antidepressants". Journal of Clinical Psychopharmacology. 29 (2): 157–64. doi:10.1097/jcp.0b013e31819c76e9. PMID 19512977.
- ^ Waldinger, MD (2015). "Psychiatric disorders and sexual dysfunction". Handbook of Clinical Neurology / edited by David B. Vodušek and François Boller. Handbook of Clinical Neurology. 130: 469–89. doi:10.1016/B978-0-444-63247-0.00027-4. ISBN 9780444632470. PMID 26003261.
- ^ http://pi.lilly.com/us/prozac.pdf Page 14.
- ^ Reisman, Yacov (2017-06-19). "Sexual Consequences of Post-SSRI Syndrome". Sexual Medicine Reviews. doi:10.1016/j.sxmr.2017.05.002. ISSN 2050-0521. PMID 28642048.
- ^ American Psychiatric Association (2013), Diagnostic and Statistical Manual of Mental Disorders (5th ed.), Arlington: American Psychiatric Publishing, p. 449, ISBN 0890425558
- ^ Gitlin, M. J. (1994-09-01). "Psychotropic medications and their effects on sexual function: diagnosis, biology, and treatment approaches". The Journal of Clinical Psychiatry. 55 (9): 406–413. ISSN 0160-6689. PMID 7929021.
- ^ Balon R (2006). "SSRI-Associated Sexual Dysfunction". The American Journal of Psychiatry. 163 (9): 1504–9; quiz 1664. doi:10.1176/appi.ajp.163.9.1504. PMID 16946173.
- ^ Clayton, Anita H. (2003). "Antidepressant-Associated Sexual Dysfunction: A Potentially Avoidable Therapeutic Challenge". Primary Psychiatry. 10 (1): 55–61.
- ^ Kanaly KA, Berman JR (December 2002). "Sexual side effects of SSRI medications: potential treatment strategies for SSRI-induced female sexual dysfunction". Current Women's Health Reports. 2 (6): 409–16. PMID 12429073.
- ^ Koyuncu H, Serefoglu EC, Ozdemir AT, Hellstrom WJ (September 2012). "Deleterious effects of selective serotonin reuptake inhibitor treatment on semen parameters in patients with lifelong premature ejaculation". Int. J. Impot. Res. 24 (5): 171–3. doi:10.1038/ijir.2012.12. PMID 22573230.
- ^ Podolej, GS; Babcock, C (January 2017). "Emergency Department Management Of Priapism". Emergency medicine practice. 19 (1): 1–16. PMID 28027457.
- ^ Oh SW, Kim J, Myung SK, Hwang SS, Yoon DH (Mar 20, 2014). "Antidepressant Use and Risk of Coronary Heart Disease: Meta-Analysis of Observational Studies". British Journal of Clinical Pharmacology. 78 (4): 727–37. doi:10.1111/bcp.12383. PMC 4239967
 . PMID 24646010.
. PMID 24646010. - ^ Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, Mogun H, Levin R, Kowal M, Setoguchi S, Hernández-Díaz S (2014). "Antidepressant Use in Pregnancy and the Risk of Cardiac Defects". New England Journal of Medicine. 370 (25): 2397–2407. doi:10.1056/NEJMoa1312828. ISSN 0028-4793. PMC 4062924
 . PMID 24941178.
. PMID 24941178. - ^ Goldberg RJ (1998). "Selective serotonin reuptake inhibitors: infrequent medical adverse effects". Archives of Family Medicine. 7 (1): 78–84. doi:10.1001/archfami.7.1.78. PMID 9443704.
- ^ FDA. "FDA Drug Safety".
- ^ Citalopram and escitalopram: QT interval prolongation—new maximum daily dose restrictions (including in elderly patients), contraindications, and warnings. From Medicines and Healthcare Products Regulatory Agency. Article date: December 2011
- ^ "Clinical and ECG Effects of Escitalopram Overdose" (PDF). Retrieved 2012-09-23.
- ^ Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V (Jun 1999). "Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any?". Current Medicinal Chemistry. 6 (6): 469–80. PMID 10213794.
- ^ a b Weinrieb RM, Auriacombe M, Lynch KG, Lewis JD (March 2005). "Selective serotonin re-uptake inhibitors and the risk of bleeding". Expert Opinion on Drug Safety. 4 (2): 337–44. doi:10.1517/14740338.4.2.337. PMID 15794724.
- ^ a b Taylor, D; Carol, P; Shitij, K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 9780470979693.
- ^ Andrade C, Sandarsh S, Chethan KB, Nagesh KS (December 2010). "Serotonin Reuptake Inhibitor Antidepressants and Abnormal Bleeding: A Review for Clinicians and a Reconsideration of Mechanisms". The Journal of Clinical Psychiatry. 71 (12): 1565–1575. doi:10.4088/JCP.09r05786blu. PMID 21190637.
- ^ a b de Abajo FJ, García-Rodríguez LA (July 2008). "Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents". Archives of General Psychiatry. 65 (7): 795–803. doi:10.1001/archpsyc.65.7.795. PMID 18606952.
- ^ Hackam DG, Mrkobrada M (2012). "Selective serotonin reuptake inhibitors and brain hemorrhage: a meta-analysis". Neurology. 79 (18): 1862–5. doi:10.1212/WNL.0b013e318271f848. PMID 23077009.
- ^ Serebruany VL (February 2006). "Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something?". The American Journal of Medicine. 119 (2): 113–6. doi:10.1016/j.amjmed.2005.03.044. PMID 16443409.
- ^ Halperin D, Reber G (2007). "Influence of antidepressants on hemostasis". Dialogues in Clinical Neuroscience. 9 (1): 47–59. PMC 3181838
 . PMID 17506225.
. PMID 17506225. - ^ Andrade C, Sandarsh S, Chethan KB, Nagesh KS (2010). "Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms". The Journal of Clinical Psychiatry. 71 (12): 1565–75. doi:10.4088/JCP.09r05786blu. PMID 21190637.
- ^ de Abajo FJ (2011). "Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients". Drugs & Aging. 28 (5): 345–67. doi:10.2165/11589340-000000000-00000. PMID 21542658.
- ^ Eom, CS; Lee, HK; Ye, S; Park, SM; Cho, KH (May 2012). "Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis". Journal of Bone and Mineral Research. 27 (5): 1186–95. doi:10.1002/jbmr.1554. PMID 22258738.
- ^ Bruyère, O; Reginster, JY (February 2015). "Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome". Endocrine. 48 (1): 65–8. doi:10.1007/s12020-014-0357-0. PMID 25091520.
- ^ Hant, FN; Bolster, MB (April 2016). "Drugs that may harm bone: Mitigating the risk". Cleveland Clinic journal of medicine. 83 (4): 281–8. doi:10.3949/ccjm.83a.15066. PMID 27055202.
- ^ Fernandes, BS; Hodge, JM; Pasco, JA; Berk, M; Williams, LJ (January 2016). "Effects of Depression and Serotonergic Antidepressants on Bone: Mechanisms and Implications for the Treatment of Depression". Drugs & aging. 33 (1): 21–5. doi:10.1007/s40266-015-0323-4. PMID 26547857.
- ^ Nyandege, AN; Slattum, PW; Harpe, SE (April 2015). "Risk of fracture and the concomitant use of bisphosphonates with osteoporosis-inducing medications". The Annals of pharmacotherapy. 49 (4): 437–47. doi:10.1177/1060028015569594. PMID 25667198.
- ^ a b Warden, SJ; Fuchs, RK (October 2016). "Do Selective Serotonin Reuptake Inhibitors (SSRIs) Cause Fractures?". Current osteoporosis reports. 14 (5): 211–8. doi:10.1007/s11914-016-0322-3. PMID 27495351.
- ^ Winterhalder, L; Eser, P; Widmer, J; Villiger, PM; Aeberli, D (December 2012). "Changes in volumetric BMD of radius and tibia upon antidepressant drug administration in young depressive patients". Journal of musculoskeletal & neuronal interactions. 12 (4): 224–9. PMID 23196265.
- ^ a b "PsychiatryOnline | APA Practice Guidelines | Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition".[permanent dead link]
- ^ Renoir T (2013). "Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved". Frontiers in Pharmacology. 4: 45. doi:10.3389/fphar.2013.00045. PMC 3627130
 . PMID 23596418.
. PMID 23596418. - ^ Volpi-Abadie, Jacqueline; Kaye, Adam M.; Kaye, Alan David (2013). "Serotonin syndrome". The Ochsner Journal. 13 (4): 533–540. ISSN 1524-5012. PMC 3865832
 . PMID 24358002.
. PMID 24358002. - ^ Boyer, Edward (2005). "The Serotonin Syndrome" (PDF). New England Journal of Medicine. doi:10.1056/nejmra041867.
- ^ https://jamanetwork.com/journals/jamaneurology/fullarticle/2673391?cmp=1
- ^ a b Stone MB, Jones ML (2006-11-17). "Clinical review: relationship between antidepressant drugs and suicidal behavior in adults" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 11–74. Retrieved 2007-09-22.
- ^ Levenson M, Holland C (2006-11-17). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 75–140. Retrieved 2007-09-22.
- ^ Olfson M, Marcus SC, Shaffer D (August 2006). "Antidepressant drug therapy and suicide in severely depressed children and adults: A case-control study". Archives of General Psychiatry. 63 (8): 865–72. doi:10.1001/archpsyc.63.8.865. PMID 16894062.
- ^ Hammad TA (2004-08-16). "Review and evaluation of clinical data. Relationship between psychiatric drugs and pediatric suicidal behavior" (PDF). FDA. pp. 42; 115. Retrieved 2008-05-29.
- ^ "Antidepressant Use in Children, Adolescents, and Adults".
- ^ "FDA Medication Guide for Antidepressants". Retrieved 2014-06-05.
- ^ a b Cox, Georgina R.; Callahan, Patch; Churchill, Rachel; Hunot, Vivien; Merry, Sally N.; Parker, Alexandra G.; Hetrick, Sarah E. (2014-11-30). "Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents". The Cochrane Database of Systematic Reviews (11): CD008324. doi:10.1002/14651858.CD008324.pub3. ISSN 1469-493X. PMID 25433518.
- ^ "www.nice.org.uk" (PDF).
- ^ Meta-Analysis of Aggression and/or Hostility-Related Events in Children and Adolescents Treated with Fluoxetine Compared with Placebo Journal of Child and Adolescent Psychopharmacology. October 2007; 17(5) 713–718. doi:10.1089/cap.2006.0138.
- ^ Gibbons RD, Hur K, Bhaumik DK, Mann JJ (November 2006). "The relationship between antidepressant prescription rates and rate of early adolescent suicide". The American Journal of Psychiatry. 163 (11): 1898–904. doi:10.1176/appi.ajp.163.11.1898. PMID 17074941.
- ^ "Report of the CSM expert working group on the safety of selective serotonin reuptake inhibitor antidepressants" (PDF). MHRA. 2004-12-01. Retrieved 2007-09-25.
- ^ "Selective Serotonin Reuptake Inhibitors (SSRIs): Overview of regulatory status and CSM advice relating to major depressive disorder (MDD) in children and adolescents including a summary of available safety and efficacy data". MHRA. 2005-09-29. Archived from the original on 2008-08-02. Retrieved 2008-05-29.
- ^ Gunnell D, Saperia J, Ashby D (February 2005). "Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA's safety review". BMJ (Clinical Research Ed.). 330 (7488): 385. doi:10.1136/bmj.330.7488.385. PMC 549105
 . PMID 15718537.
. PMID 15718537. - ^ Fergusson D, Doucette S, Glass KC, Shapiro S, Healy D, Hebert P, Hutton B (February 2005). "Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials". BMJ (Clinical Research Ed.). 330(7488): 396. doi:10.1136/bmj.330.7488.396. PMC 549110
 . PMID 15718539.
. PMID 15718539. - ^ Rihmer Z, Akiskal H (August 2006). "Do antidepressants t(h)reat(en) depressives? Toward a clinically judicious formulation of the antidepressant-suicidality FDA advisory in light of declining national suicide statistics from many countries". Journal of Affective Disorders. 94(1–3): 3–13. doi:10.1016/j.jad.2006.04.003. PMID 16712945.
- ^ Hall WD, Lucke J (2006). "How have the selective serotonin reuptake inhibitor antidepressants affected suicide mortality?". The Australian and New Zealand Journal of Psychiatry. 40 (11–12): 941–50. doi:10.1111/j.1440-1614.2006.01917.x. PMID 17054562.
- ^ Malm H (December 2012). "Prenatal exposure to selective serotonin reuptake inhibitors and infant outcome". Therapeutic Drug Monitoring. 34 (6): 607–14. doi:10.1097/FTD.0b013e31826d07ea. PMID 23042258.
- ^ Rahimi R, Nikfar S, Abdollahi M (2006). "Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials". Reproductive Toxicology (Elmsford, N.Y.). 22 (4): 571–575. doi:10.1016/j.reprotox.2006.03.019. PMID 16720091.
- ^ a b Nikfar S, Rahimi R, Hendoiee N, Abdollahi M (2012). "Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: A systematic review and updated meta-analysis". Daru : Journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 20 (1): 75. doi:10.1186/2008-2231-20-75. PMC 3556001
 . PMID 23351929.
. PMID 23351929. - ^ Eke, AC; Saccone, G; Berghella, V (November 2016). "Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and meta-analysis". BJOG : an international journal of obstetrics and gynaecology. 123 (12): 1900–1907. doi:10.1111/1471-0528.14144. PMID 27239775.
- ^ Einarson TR, Kennedy D, Einarson A (2012). "Do findings differ across research design? The case of antidepressant use in pregnancy and malformations". Journal of Population Therapeutics and Clinical Pharmacology = Journal de la Thérapeutique des Populations et de la Pharamcologie Clinique. 19 (2): e334–48. PMID 22946124.
- ^ Riggin L, Frankel Z, Moretti M, Pupco A, Koren G (April 2013). "The fetal safety of fluoxetine: a systematic review and meta-analysis". Journal of Obstetrics and Gynaecology Canada. 35 (4): 362–9. doi:10.1016/S1701-2163(15)30965-8. PMID 23660045.
- ^ Koren G, Nordeng HM (February 2013). "Selective serotonin reuptake inhibitors and malformations: case closed?". Seminars in Fetal & Neonatal Medicine. 18 (1): 19–22. doi:10.1016/j.siny.2012.10.004. PMID 23228547.
- ^ "Breastfeeding Update: SDCBC's quarterly newsletter". Breastfeeding.org. Archived from the original on February 25, 2009. Retrieved 2010-07-10.
- ^ "Using Antidepressants in Breastfeeding Mothers". kellymom.com. Archived from the original on 2010-09-23. Retrieved 2010-07-10.
- ^ Gentile S, Rossi A, Bellantuono C (2007). "SSRIs during breastfeeding: spotlight on milk-to-plasma ratio". Archives of Women's Mental Health. 10 (2): 39–51. doi:10.1007/s00737-007-0173-0. PMID 17294355.
- ^ Fenger-Grøn J, Thomsen M, Andersen KS, Nielsen RG (September 2011). "Paediatric outcomes following intrauterine exposure to serotonin reuptake inhibitors: a systematic review". Danish Medical Bulletin. 58 (9): A4303. PMID 21893008.
- ^ Kieviet N, Dolman KM, Honig A (2013). "The use of psychotropic medication during pregnancy: how about the newborn?". Neuropsychiatric Disease and Treatment. 9: 1257–1266. doi:10.2147/NDT.S36394. PMC 3770341
 . PMID 24039427.
. PMID 24039427. - ^ "Persistent Newborn Pulmonary Hypertension".
- ^ Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A, Ross LE (2014). "Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis". BMJ (Clinical Research Ed.). 348: f6932. doi:10.1136/bmj.f6932. PMC 3898424
 . PMID 24429387.
. PMID 24429387. - ^ 't Jong GW, Einarson T, Koren G, Einarson A (November 2012). "Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review". Reproductive Toxicology. 34 (3): 293–7. doi:10.1016/j.reprotox.2012.04.015. PMID 22564982.
- ^ Gentile S. (2015). "Prenatal antidepressant exposure and the risk of autism spectrum disorders in children. Are we looking at the fall of Gods?". J. Affect. Disord. 182: 132–7. doi:10.1016/j.jad.2015.04.048. PMID 25985383. Epub 2015 May 6.
- ^ Hviid A, Melbye M, Pasternak B (19 December 2013). "Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism". The New England Journal of Medicine. 369 (25): 2406–2415. doi:10.1056/NEJMoa1301449. PMID 24350950. Retrieved 18 December 2013.
- ^ a b Heli Malm, Alan S. Brown et al. (2016). "Gestational Exposure to Selective Serotonin Reuptake Inhibitors and Offspring Psychiatric Disorders: A National Register-Based Study". Journal of the American Academy of Child & Adolescent Psychiatry. 55: 359–366. doi:10.1016/j.jaac.2016.02.013.
- ^ a b c Isbister GK, Bowe SJ, Dawson A, Whyte IM (2004). "Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose". Journal of Toxicology. Clinical Toxicology. 42 (3): 277–85. doi:10.1081/CLT-120037428. PMID 15362595.
- ^ Borys DJ, Setzer SC, Ling LJ, Reisdorf JJ, Day LC, Krenzelok EP (1992). "Acute fluoxetine overdose: a report of 234 cases". The American Journal of Emergency Medicine. 10 (2): 115–20. doi:10.1016/0735-6757(92)90041-U. PMID 1586402.
- ^ Oström M, Eriksson A, Thorson J, Spigset O (1996). "Fatal overdose with citalopram". Lancet. 348 (9023): 339–40. doi:10.1016/S0140-6736(05)64513-8. PMID 8709713.
- ^ Sporer KA (1995). "The serotonin syndrome. Implicated drugs, pathophysiology and management". Drug Safety. 13 (2): 94–104. doi:10.2165/00002018-199513020-00004. PMID 7576268.
- ^ White N, Litovitz T, Clancy C (December 2008). "Suicidal antidepressant overdoses: a comparative analysis by antidepressant type" (PDF). Journal of Medical Toxicology. 4 (4): 238–250. doi:10.1007/BF03161207. PMC 3550116
 . PMID 19031375.
. PMID 19031375. - ^ Ener RA, Meglathery SB, Van Decker WA, Gallagher RM (March 2003). "Serotonin Syndrome and Other Serotonergic Disorders". Pain Medicine. 4 (1): 63–74. doi:10.1046/j.1526-4637.2003.03005.x. PMID 12873279.
- ^ Boyer EW, Shannon M; Shannon, M (2005). "The serotonin syndrome" (PDF). The New England Journal of Medicine. 352 (11): 1112–1120. doi:10.1056/NEJMra041867. PMID 15784664.
- ^ Solomon H. Snyder (2011). "Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans". Proceedings of the National Academy of Sciences. 108: 9262–9267. doi:10.1073/pnas.1104836108. PMC 3107316
 . PMID 21518864. Retrieved 2012-09-23.
. PMID 21518864. Retrieved 2012-09-23. - ^ Brunton, L; Chabner, B; Knollman, B (2010). Goodman and Gilman’s The Pharmacological Basis of Therapeutics (12th ed.). McGraw Hill Professional. ISBN 978-0071624428.
- ^ Ciraulo, DA; Shader, RI (2011). Pharmacotherapy of Depression (2nd ed.). Springer. p. 49. doi:10.1007/978-1-60327-435-7. ISBN 978-1-60327-435-7.
- ^ a b c d Shelton R. C. (2009). "Serotonin norepinephrine reuptake inhibitors: similarities and differences". Primary Psychiatry. 16 (4): 25.
- ^ Derek G. Waller; Tony Sampson (4 June 2017). Medical Pharmacology and Therapeutics E-Book. Elsevier Health Sciences. pp. 302–. ISBN 978-0-7020-7190-4.
- ^ Susan G. Kornstein; Anita H. Clayton (5 May 2010). Women's Mental Health, An Issue of Psychiatric Clinics – E-Book. Elsevier Health Sciences. pp. 389–. ISBN 1-4557-0061-4.
- ^ Bruno A, Morabito P, Spina E, Muscatello MR (2016). "The Role of Levomilnacipran in the Management of Major Depressive Disorder: A Comprehensive Review". Curr Neuropharmacol. 14 (2): 191–9. doi:10.2174/1570159x14666151117122458. PMC 4825949
 . PMID 26572745.
. PMID 26572745. - ^ a b Mandrioli R, Protti M, Mercolini L (2017). "New-Generation, non-SSRI Antidepressants: Therapeutic Drug Monitoring and Pharmacological Interactions. Part 1: SNRIs, SMSs, SARIs". Curr. Med. Chem. 24. doi:10.2174/0929867324666170712165042. PMID 28707591.
- ^ a b Frank J. Ayd (2000). Lexicon of Psychiatry, Neurology, and the Neurosciences. Lippincott Williams & Wilkins. pp. 581–. ISBN 978-0-7817-2468-5.
- ^ a b Progress in Drug Research. Birkhäuser. 6 December 2012. pp. 80–82. ISBN 978-3-0348-8391-7.
- ^ a b Moltzen EK, Bang-Andersen B (2006). "Serotonin reuptake inhibitors: the corner stone in treatment of depression for half a century—a medicinal chemistry survey". Curr Top Med Chem. 6 (17): 1801–23. doi:10.2174/156802606778249810. PMID 17017959.
- ^ a b Haddad, Peter M. (2000). "Selective Serotonin Reuptake Inhibitors (SSRIs) Past, Present and Future. Edited by S. Clare Standford, R.G. Landes Company, Austin, Texas, USA, 1999". Human Psychopharmacology: Clinical and Experimental. 15 (6): 205–207. doi:10.1002/1099-1077(200008)15:6<471::AID-HUP211>3.0.CO;2-4. ISBN 1-57059-649-2. ISSN 0885-6222.
- ^ a b Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- ^ a b c Hellbom E (2006). "Chlorpheniramine, selective serotonin-reuptake inhibitors (SSRIs) and over-the-counter (OTC) treatment". Med. Hypotheses. 66 (4): 689–90. doi:10.1016/j.mehy.2005.12.006. PMID 16413139.
- ^ Goodman, Louis S. (Louis Sanford); Brunton, Laurence L.; Chabner, Bruce.; Knollmann, Björn C. (2001). Goodman and Gilman's pharmacological basis of therapeutics. New York: McGraw-Hill. pp. 459–461. ISBN 0-07-162442-2.
- ^ a b Kolb, Bryan and Wishaw Ian. An Introduction to Brain and Behavior. New York: Worth Publishers 2006, Print.
- ^ a b c d e f g Hindmarch I, Hashimoto K (2010). "Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered". Hum Psychopharmacol. 25 (3): 193–200. doi:10.1002/hup.1106. PMID 20373470.
- ^ a b c d e f g Albayrak, Yakup; Hashimoto, Kenji (2017). "Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders". Advances in Experimental Medicine and Biology. 964: 153–161. doi:10.1007/978-3-319-50174-1_11. ISSN 0065-2598.
- ^ Kohler, Ole; Krogh, Jesper; Mors, Ole; Eriksen Benros, Michael (26 August 2016). "Inflammation in Depression and the Potential for Anti-Inflammatory Treatment". Current Neuropharmacology. 14 (7): 732–742. doi:10.2174/1570159X14666151208113700. PMC 5050394
 .
. - ^ Köhler, Stephan; Cierpinsky, Katharina; Kronenberg, Golo; Adli, Mazda (January 2016). "The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants". Journal of Psychopharmacology. 30 (1): 13–22. doi:10.1177/0269881115609072.
- ^ Köhler, Cristiano A.; Freitas, Thiago H.; Stubbs, Brendon; Maes, Michael; Solmi, Marco; Veronese, Nicola; de Andrade, Nayanna Q.; Morris, Gerwyn; Fernandes, Brisa S.; Brunoni, André R.; Herrmann, Nathan; Raison, Charles L.; Miller, Brian J.; Lanctôt, Krista L.; Carvalho, André F. (13 June 2017). "Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis". Molecular Neurobiology. doi:10.1007/s12035-017-0632-1.
- ^ Więdłocha, Magdalena; Marcinowicz, Piotr; Krupa, Renata; Janoska-Jaździk, Marlena; Janus, Marta; Dębowska, Weronika; Mosiołek, Anna; Waszkiewicz, Napoleon; Szulc, Agata (April 2017). "Effect of antidepressant treatment on peripheral inflammation markers – A meta-analysis". Progress in Neuro-Psychopharmacology and Biological Psychiatry. doi:10.1016/j.pnpbp.2017.04.026.
- ^ Vogelzangs, N; Duivis, H E; Beekman, A T F; Kluft, C; Neuteboom, J; Hoogendijk, W; Smit, J H; de Jonge, P; Penninx, B W J H (February 2012). "Association of depressive disorders, depression characteristics and antidepressant medication with inflammation". Translational Psychiatry. 2 (2): e79. doi:10.1038/tp.2012.8.
- ^ a b Kalkman, Hans O.; Feuerbach, Dominik (July 2016). "Antidepressant therapies inhibit inflammation and microglial M1-polarization". Pharmacology & Therapeutics. 163: 82–93. doi:10.1016/j.pharmthera.2016.04.001.
- ^ a b Nazimek, Katarzyna; Strobel, Spencer; Bryniarski, Paweł; Kozlowski, Michael; Filipczak-Bryniarska, Iwona; Bryniarski, Krzysztof (June 2017). "The role of macrophages in anti-inflammatory activity of antidepressant drugs". Immunobiology. 222 (6): 823–830. doi:10.1016/j.imbio.2016.07.001.
- ^ a b Gobin, Veerle; Van Steendam, Katleen; Denys, Damiaan; Deforce, Dieter (May 2014). "Selective serotonin reuptake inhibitors as a novel class of immunosuppressants". International Immunopharmacology. 20 (1): 148–156. doi:10.1016/j.intimp.2014.02.030.
- ^ Rasmussen-Torvik LJ, McAlpine DD (2007). "Genetic screening for SSRI drug response among those with major depression: great promise and unseen perils". Depression and Anxiety. 24 (5): 350–7. doi:10.1002/da.20251. PMID 17096399.
- ^ Anderson IM (April 2000). "Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability". Journal of Affective Disorders. 58 (1): 19–36. doi:10.1016/S0165-0327(99)00092-0. PMID 10760555.
- ^ Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R (January 2008). "Selective publication of antidepressant trials and its influence on apparent efficacy". The New England Journal of Medicine. 358 (3): 252–60. doi:10.1056/NEJMsa065779. PMID 18199864.
- ^ Healy D; Aldred G (2005). "Antidepressant drug use and the risk of suicide" (PDF). International Review of Psychiatry. 17: 163–172. doi:10.1080/09540260500071624. Archived from the original (PDF) on 2013-10-21.
- ^ Lapierre YD (September 2003). "Suicidality with selective serotonin reuptake inhibitors: Valid claim?". J Psychiatry Neurosci. 28 (5): 340–7. PMC 193980
 . PMID 14517577.
. PMID 14517577. - ^ Khan A, Khan S, Kolts R, Brown WA (2003). "Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports". Am. J. Psychiatry. 160: 790–2. doi:10.1176/appi.ajp.160.4.790. PMID 12668373.
- ^ Kaizar EE, Greenhouse JB, Seltman H, Kelleher K (2006). "Do antidepressants cause suicidality in children? A Bayesian meta-analysis". Clin. Trials. 3 (2): 73–90; discussion 91–8. doi:10.1191/1740774506cn139oa. PMID 16773951.
- ^ Gibbons RD, Brown CH, Hur K, Davis J, Mann JJ (June 2012). "Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine". Arch. Gen. Psychiatry. 69 (6): 580–7. doi:10.1001/archgenpsychiatry.2011.2048. PMC 3367101
 . PMID 22309973.
. PMID 22309973.